Medication-Induced Pancreatitis Risk Assessor
Assessment Tool
Typically 7-14 days after starting new medication (may vary by drug class)
Normal range: 15-70 U/L. Severe pancreatitis likely when ≥3× normal (e.g., 1,250 U/L)
Click to select relevant medication classes
Risk Assessment
When a prescription drug turns the pancreas into a ticking time‑bomb, the stakes are high. Medication‑induced severe pancreatitis is a rare but life‑threatening reaction that demands swift recognition, immediate drug withdrawal, and aggressive supportive care.
Why drug‑induced pancreatitis matters
Even though medications cause only 1.4‑3.6% of all acute pancreatitis cases, they account for roughly 20% of the severe forms, according to the Cleveland Clinic’s 2023 data. Mortality jumps to 15‑30% in severe cases-far higher than the 10‑12% seen with gallstone pancreatitis. The upside? If caught early, stopping the offending drug can reverse the damage in two‑thirds of patients.
How to spot the warning signs
The classic trio-upper abdominal pain radiating to the back, nausea, and vomiting-appears in drug‑related cases, but the timeline is the real clue. Symptoms usually develop 7‑14 days after starting a new medication, although some agents (like statins) can trigger pain after years of uneventful use.
- Pain pattern: sudden, constant, often worse after meals; may awaken you at night.
- Lab red flag: serum lipase three times the upper limit (e.g., 1,250 U/L when normal < 60).
- Systemic signs: fever >38 °C, heart rate >90 bpm, respiratory rate >20, or white‑blood‑cell count >12,000 mm³ indicating SIRS.
Imaging with contrast‑enhanced CT can reveal pancreatic necrosis covering >30% of the gland-one of the Revised Atlanta Classification criteria for severe disease.
Medication classes with the strongest evidence
| Drug class | Typical latency (days) | Mechanism (proposed) | Severe outcome rate |
|---|---|---|---|
| ACE inhibitors (lisinopril, enalapril) | 5‑10 | Direct acinar toxicity | 5‑7% |
| Didanosine (HAART) | 14‑21 | Metabolic disturbance | 12‑15% |
| Furosemide & thiazides | 7‑14 | Electrolyte‑driven ductal dysfunction | 6‑8% |
| Exenatide & sitagliptin | 10‑20 | Immune‑mediated hypersensitivity | 9‑11% |
| Valproic acid | 3‑7 | Direct toxic injury | 22% |
| Azathioprine | 4‑10 | Immune reaction + metabolite toxicity | 18% |
| Statins (simvastatin, atorvastatin) | 30‑60 | Unclear; possibly idiosyncratic | 5‑7% |
| Oral contraceptives (ethinyl estradiol) | 21‑35 | Hormonal modulation of pancreatic secretions | 4‑6% |
Note that these percentages come from pooled data across multiple centers and reflect the proportion of severe outcomes among documented drug‑induced cases.
Pathophysiology in plain language
Scientists still debate why some pills hurt the pancreas. The three leading ideas are:
- Direct toxicity: The drug or its metabolite damages acinar cells, the pancreas’s enzyme‑producing units.
- Immune‑mediated hypersensitivity: The body misidentifies the medication as a threat, unleashing inflammation that spills into the pancreas.
- Metabolic disruption: Changes in calcium, bicarbonate, or ductal flow create a buildup of activated enzymes that start digesting the organ itself.
In reality, most cases involve a mix of these mechanisms, which makes prediction difficult.
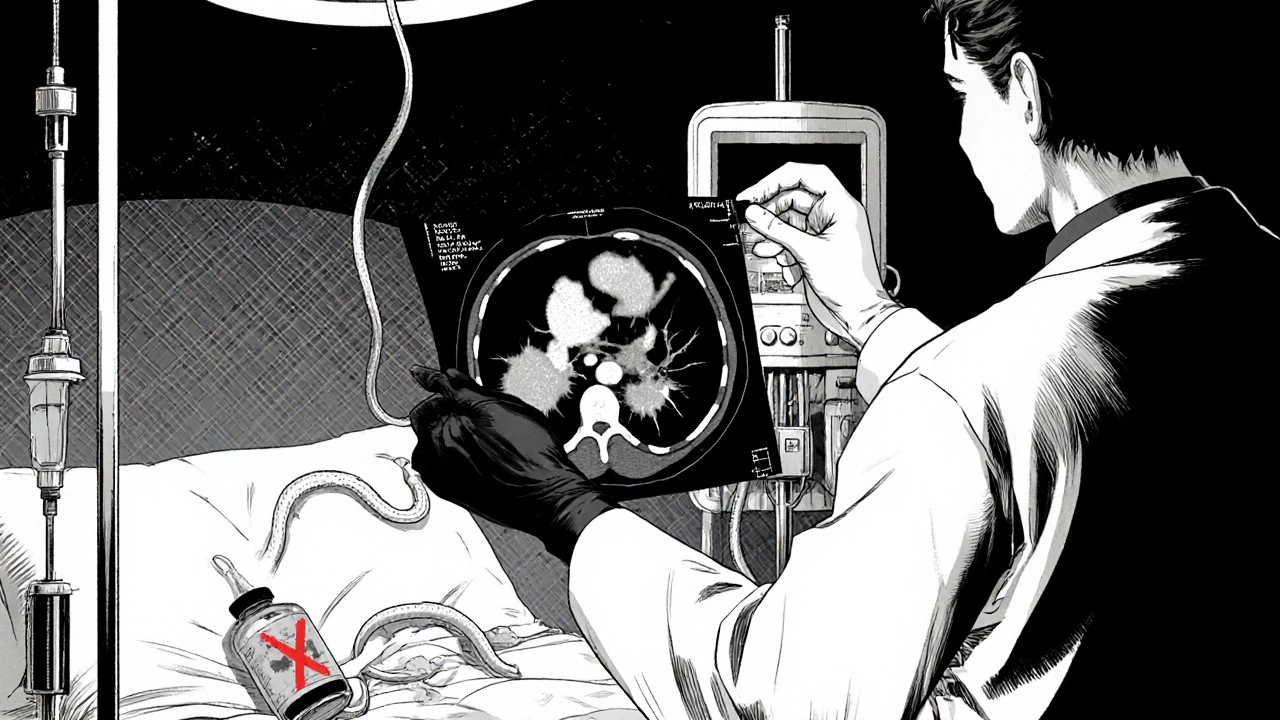
Step‑by‑step management protocol
Time is the most critical factor. The following algorithm is what most tertiary centers follow today.
- Recognize and confirm: Order serum lipase, CBC, and urgent contrast‑CT. If lipase ≥3× normal and imaging shows inflammation, move to step 2.
- Stop the suspect medication within 24 hours: Delayed withdrawal adds a 37% increase in complications (2022 meta‑analysis).
- IV fluid resuscitation: 250‑500 mL/hour of isotonic crystalloid; titrate to keep hematocrit 35‑44%.
- Pain control: Acetaminophen 1 g IV q6h; if inadequate, add morphine 2‑4 mg IV q2‑3h, monitoring respiratory status.
- Nutritional support: Keep NPO for the first 24‑48 h, then start enteral feeding via nasojejunal tube, targeting 20‑25 kcal/kg/day by day 3.
- Antibiotics only if infected necrosis is proven: Meropenem 1 g IV q8h.
- Organ‑failure monitoring: Serial SOFA scores; if failure persists >48 h, consider ICU transfer.
- Re‑assessment of etiology: If no clear offending drug, broaden work‑up (gallstones, hypertriglyceridemia, genetic).
Early involvement of a gastroenterology team improves outcomes-most studies show a 15% reduction in mortality when a specialist is consulted within the first 12 hours.
Distinguishing drug‑induced from other causes
Gallstone pancreatitis typically peaks within 72 hours and resolves in 80% of cases. Alcohol‑related pancreatitis is often recurrent. The key discriminators for drug‑induced disease are:
- Temporal link to medication start (usually <4 weeks).
- Absence of gallstones on ultrasound.
- Persistence of high lipase despite aggressive fluid therapy.
In ambiguous cases, the American Gastroenterological Association suggests a “probable” rating when symptoms appear <4 weeks after drug initiation and improve within 8 weeks of stopping the drug.
Real‑world stories that illustrate the stakes
Jane, 45, had been on lisinopril for six months when she woke at 3 am with crushing upper‑abdominal pain. Lipase shot up to 1,250 U/L, CT showed pancreatic edema, and after the drug was stopped she was pain‑free in three days. Her story mirrors a 2022 Drugs.com forum thread where 68% of patients reported delayed diagnosis because clinicians first blamed gastritis.
Another case involved a 62‑year‑old man on simvastatin for three years. Lipase peaked at 2,800 U/L, and after discontinuing the statin his labs normalized in 72 hours. These anecdotes highlight that even long‑term users aren’t immune.

Prevention strategies for clinicians
Because polypharmacy is the biggest risk factor (average 5.2 meds in patients >60 y), hospitals now use EHR alerts. When a high‑risk drug is prescribed alongside another known culprit, the system flags the patient for baseline lipase testing. The CMS rule of 2021 that denies reimbursement for preventable severe pancreatitis has spurred 78% of academic centers to adopt such alerts.
Pharmacogenetic testing is emerging, especially for azathioprine. Patients with TPMT deficiency have a 3‑fold higher chance of developing pancreatitis, making pre‑emptive testing cost‑effective according to the 2023 Nature Reviews editorial.
Future directions and research gaps
The NIH‑funded Drug‑Induced Pancreatitis Registry (DIPR) aims to collect uniform causality data across 28 centers. Early results suggest that a standardized scoring system improves diagnostic confidence by 22% over the Naranjo scale alone.
Immunotherapy agents such as ipilimumab‑nivolumab now carry a 9.2% pancreatitis incidence, with a third becoming severe. As oncology expands, clinicians will need integrated oncology‑gastroenterology pathways.
Finally, a 2023 consensus predicts a 25% rise in severe drug‑induced cases over the next decade, driven by aging populations and expanding medication indications. Ongoing education, alert systems, and early‑testing protocols will be the front line of defense.
Take‑away checklist for patients and providers
- Ask for baseline lipase when starting a high‑risk medication.
- Report any new, persistent upper‑abdominal pain promptly.
- If pancreatitis is diagnosed, discontinue the suspect drug within 24 hours.
- Initiate aggressive IV fluids and pain control per ACG guidelines.
- Consider early gastroenterology consult and repeat imaging at 48‑72 hours.
What are the most common medications that cause severe pancreatitis?
The highest‑risk classes are ACE inhibitors, didanosine, furosemide (and other thiazides), GLP‑1 agonists such as exenatide, valproic acid, azathioprine, and certain statins. Each carries a different latency and severity profile, but all can trigger severe disease if the pancreas reacts.
How quickly should the offending drug be stopped?
Within 24 hours of suspecting drug‑induced pancreatitis. Delays beyond a day raise the risk of complications by about 37%.
Can drug‑induced pancreatitis be prevented?
Prevention focuses on careful medication review, especially in older adults on multiple drugs. Baseline lipase testing, pharmacogenetic screening for high‑risk agents, and EHR alerts for known culprit combinations reduce incidence.
Is a lipase test enough to diagnose severe pancreatitis?
A lipase three‑fold above normal supports the diagnosis, but severity requires imaging (CT or MRI) to assess necrosis, plus clinical criteria like persistent organ failure per the Revised Atlanta Classification.
What supportive care is critical in the first 48 hours?
Aggressive IV fluid resuscitation (250‑500 mL/h), pain control (acetaminophen then low‑dose opioids), and early nutritional assessment (enteral feeding if oral intake is impossible). Monitoring for organ failure with serial SOFA scores is also essential.




12 Comments
Carla Taylor
October 24, 2025 AT 16:44 PMGot the gist and feeling hopeful about catching these meds early
Mary Mundane
October 25, 2025 AT 20:46 PMThe focus on obscure meds seems overstated and wastes time
Jacqueline Galvan
October 27, 2025 AT 00:06 AMWhile the prevalence of drug‑induced pancreatitis is low, the mortality in severe cases justifies vigilance. The algorithm outlined aligns with current guidelines from the AGA and IAP. Early discontinuation of the suspected agent within 24 hours markedly reduces the risk of necrosis. Fluid resuscitation should target a hematocrit of 35‑44 % to avoid hypoperfusion. Pain control using acetaminophen first, followed by opioids, minimizes respiratory depression when titrated carefully. Nutritional support via early enteral feeding improves gut barrier function and reduces infectious complications. Imaging with contrast‑enhanced CT after 48 hours helps differentiate sterile from infected necrosis. Consultation with a gastroenterology specialist within the first half‑day has been shown to cut mortality by roughly fifteen percent. Ongoing monitoring of SOFA scores ensures timely ICU escalation if organ failure persists.
Teya Arisa
October 28, 2025 AT 05:16 AMThank you for sharing this comprehensive guide; it is both thorough and actionable 😊
Maintaining a high index of suspicion can truly save lives, especially when dealing with medications like ACE inhibitors or valproic acid. Prompt cessation, aggressive fluid therapy, and early specialist involvement remain the cornerstones of management. I appreciate the clear step‑by‑step approach and will incorporate it into my practice.
Amanda Vallery
October 29, 2025 AT 10:26 AMDidnt realy think statins could cause this but now i see the data
Marilyn Pientka
October 30, 2025 AT 15:36 PMIt is egregiously negligent to overlook the iatrogenic etiology when confronted with necrotic pancreatitis; pharmacovigilance protocols must be rigorously enforced to preempt such catastrophic sequelae. The pathophysiological nexus of acinar cytotoxicity, immunologic hypersensitivity, and metabolic dysregulation constitutes a triad that warrants immediate multidisciplinary intervention.
Jordan Levine
October 31, 2025 AT 20:46 PMWow, this reads like a ticking time‑bomb scenario for anyone on pills! 💥💊 If you feel that gnawing upper‑abdominal pain, don’t wait – pull the plug on the drug and call the docs pronto!
Kathryn Rude
November 2, 2025 AT 01:56 AMHonestly its like a textbook case of over‑reacting🚀 the data isnt that black and white 😂 meds are just one piece of the puzzle but not the whole story
Ekeh Lynda
November 3, 2025 AT 07:06 AMThe clinical community has been lulled into a false sense of security by the rarity statistics and now pays insufficient attention to drug‑induced pancreatitis. The literature repeatedly emphasizes gallstone and alcohol etiologies while relegating medication triggers to footnotes. This systematic bias fuels delayed diagnosis and unnecessary morbidity. When a patient presents with epigastric pain the default workup often neglects a thorough medication reconciliation. The latency period of 7‑14 days is subtle and can be easily missed if clinicians are not vigilant. Moreover the pharmacodynamic mechanisms, whether direct acinar toxicity or immune‑mediated hypersensitivity, are rarely discussed in bedside rounds. The table presented in the article, while helpful, fails to convey the real‑world incidence of each class in diverse populations. For instance ACE inhibitors are prescribed to millions yet the severe pancreatitis rate remains under‑reported due to coding errors. Similarly, the association with valproic acid is clouded by concomitant hepatic dysfunction in epileptic patients. The omission of genetic predisposition further muddies the etiological landscape. In practice, physicians often attribute elevated lipase to unrelated causes and continue the offending drug, exacerbating tissue damage. This cascade culminates in necrotic pancreatitis, a condition with a mortality exceeding twenty percent. Early cessation of the suspect agent, as the article outlines, is not merely a recommendation but an ethical imperative. Failure to act promptly constitutes a breach of the duty of non‑maleficence. Therefore, institutions should implement mandatory alerts in electronic prescribing systems to flag high‑risk drugs when patients report abdominal symptoms. Only through such systemic safeguards can we hope to reduce the preventable toll of medication‑induced pancreatic injury.
Michelle Capes
November 4, 2025 AT 12:16 PMWow thats a lot to take in 😮 i didnt realize how many steps we miss when checking meds, thanks for breaking it down (sorry for the typos) we should definitely push for those e‑prescribing alerts!
Dahmir Dennis
November 5, 2025 AT 17:26 PMOh brilliant, another thrilling checklist for doctors who think a spreadsheet can replace clinical judgment. As if simply “stop the drug within 24 hours” magically resurrects the pancreas, ignoring the chaos that ensues when a patient is suddenly deprived of their lifelong hypertension therapy. The article’s tone suggests that all prescribers are negligent geniuses waiting for a lawsuit, which is both condescending and utterly unrealistic. Let’s not forget that many of these “high‑risk” medications are prescribed after careful risk‑benefit analysis, not tossed around like candy. The so‑called “aggressive fluid resuscitation” often leads to pulmonary edema in a population already frail from comorbidities. And the glorified “early specialist consult” is a luxury in community hospitals where gastroenterologists are a bus ride away. One would think the authors would address the socioeconomic disparities that force patients to endure suboptimal monitoring. Instead, we get a recipe for panic that might prompt clinicians to abandon life‑saving drugs out of fear. The moral panic is palpable, yet the proposed solutions are shallow. Perhaps a more nuanced discussion of individualized therapy would have been less alarmist. Until then, we’re left with a sensationalized narrative that does little but add to the noise. In short, the article trades nuance for drama, and that’s a disservice to both patients and providers.
Tammy Watkins
November 6, 2025 AT 22:36 PMWhile acknowledging the concerns raised regarding the pragmatic applicability of the protocol, it is imperative to underscore that the morbidity associated with delayed drug withdrawal remains unequivocally high. The dramatization of clinical scenarios, as noted, serves to heighten awareness among practitioners who may otherwise underestimate the gravity of medication‑induced pancreatic injury. A balanced synthesis of evidence‑based thresholds for intervention, combined with resource‑sensitive adaptations, can reconcile the tension between ideal recommendations and real‑world constraints. Moreover, the emphasis on early multidisciplinary collaboration is not merely rhetorical but grounded in outcome‑improving data from numerous tertiary centers. Therefore, rather than dismissing the algorithm as sensational, one might view it as a catalyst for systematic improvement across varied practice settings. The ultimate objective remains the preservation of patient safety through timely recognition and decisive action.